|
Fouling The deposition of any undesired material on heat transfer surfaces is called fouling. Fouling may significantly impact the thermal and mechanical performance of heat exchangers. Fouling is a dynamic phenomenon which changes with time. Fouling increases the overall thermal resistance and lowers the overall heat transfer coefficient of heat exchangers. Fouling also impedes fluid flow, accelerates corrosion and increases pressure drop across heat exchangers. Different types of fouling mechanisms have been identified. They can occur individually but often occur simultaneously. Descriptions of the most common fouling mechanisms are provided below: Scaling/Crystallization Fouling: Scaling is the most common type of fouling and is commonly associated with inverse solubility salts such as calcium carbonate (CaCO3) found in water. Reverse solubility salts become less solute as the temperature increases and thus deposit on the heat exchanger surface. Scale is difficult to remove mechanically and chemical cleaning may be required.  Particulate/Sedimentation Fouling: Sedimentation occurs when particles (e.g. dirt, sand or rust) in the solution settle and deposit on the heat transfer surface. Like scale, these deposits may be difficult to remove mechanically depending on their nature. 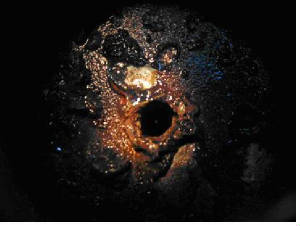  Corrosion Fouling: Results from a chemical reaction which involves the heat exchanger surface material. Many metals such as copper and aluminum form adherent oxide coatings which serve to passivate the surface and prevent further corrosion. Metal oxides which are corrosion products exhibit quite a low thermal conductivity and even relatively thin coatings of oxides may significantly affect heat exchanger performance.  Chemical Fouling: Fouling from chemical reactions in the fluid stream which result in the deposition of material on the heat exchanger surface. This type of fouling is common for chemically sensitive materials when the fluid is heated to temperatures near its decomposition (degradation) temperature. Coking of hydrocarbon material on the heat transfer surface is also a common chemical fouling problem.  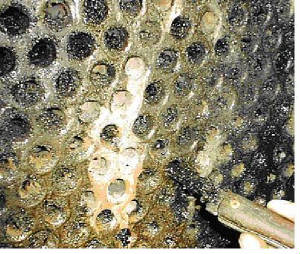 Freezing Fouling: Occurs when a portion of the hot stream is cooled to near the freezing point of one of its components. An example in refineries is when paraffin solidifies from a cooled petroleum product. Another example is freezing of polymer products on the heat exchanger surface.  Biological Fouling: Occurs when biological organisms grow on heat transfer surfaces. It is a common fouling mechanism where untreated water is used as the coolant. Problems range from algae to other microbes such as barnacles and zebra mussels. During seasons when these microbes are said to bloom, colonies several millimeters deep may grow across the surface within hours, impeding circulation near the surface wall and impacting heat transfer. 
It is important to consider fouling in the design of a heat exchanger. There are different methods to provide the added heat transfer area needed to account for the expected fouling and maximize runtime between cleaning. For shell and tube heat exchanger, the common method is to use fouling factors. For other types of heat exchangers, excess heat transfer area is often used. However, fouling is a self-fulfilling prophecy and the selection of fouling factors or excess area must be done carefully. Fouling tendencies depends on the type of heat exchanger and the fluids. During the design stage certain considerations may help minimize fouling experienced in the field:
|